
Extracellular Matrix
Provides a critical prerequisite for successful tissue regeneration, a structure designed to mimic the scaffolding of the extracellular matrix (ECM).


Provides a critical prerequisite for successful tissue regeneration, a structure designed to mimic the scaffolding of the extracellular matrix (ECM).
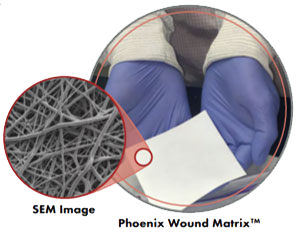
The Phoenix is fully synthetic and contains no human or animal tissue/cells, thereby eliminating the risks inherent to allogeneic and xenogeneic products.†





Dan Davis
Podiatric Surgeon
PHOENIX Wound Matrix will challenge our status quo of Cellular Tissue Products as a critical tool in our tool box for wound healing. I am seeing rapid wound closure rates on very complex wounds. PHOENIX should be considered a first-line treatment option in our fight to preserve limbs and save lives.

Dr. Eric Storts
DPM
In the OR, PHOENIX Wound Matrix, combined with PRP, provides an excellent scaffold for cellular migration, infiltration and proliferation. I have noticed a decreased inflammatory response. My patients’ wounds have remained granular and I have observed faster healing times with PHOENIX.

Denisa Riera
Podiatric Surgeon
There is a clear physiological change in the health of the tissue after first application. My patients are so pleased with the outcomes. PHOENIX is remarkable.

Frank Aviles
PT, CWS, FACCWS, CLT - Wound Care Service Line Director
With PHOENIX Wound Matrix I am seeing an immediate change in the tissue and wound environment after the first application. Wounds that were stuck in an inflammatory chronic state rapidly become healthy granular tissue and begin healing. Very impressive healing rates and outcomes.

Richard Schilling
DPM
I feel that the tissue we are repairing and regenerating with PHOENIX Wound Matrix is as strong if not stronger than before. I have seen no wound reoccurrence in my patients’ wounds.

Matthew Garoufalis
DPM, FASPS, FACFAOM, CWS, FFPM RCPS (Glasg)
As time is critically important, PHOENIX Wound Matrix should be considered as a first-line treatment option for rapid and effective wound closure. I have been able to minimize or eliminate the use of more expensive CTPs when utilizing PHOENIX Wound Matrix.
Nanofiber Solutions, LLC is a regenerative medicine company developing a new class of implants with unrivaled performance for the $20B soft tissue/organ repair/regeneration market. NFS’s technology is used to build off-the-shelf scaffolds that are critical in the development of life-saving and life-changing, tissue engineered implants. Their extensive experience working with researchers using their scaffolds in cell culture plates has given them novel insight into the behavior of adherent cells with these scaffolds. Using this knowledge NFS engineers their regenerative medicine products to meet the complex clinical requirements.